|
|
Breast Embryology
and Congenital Malformations
Congenital Deformities of the Female and Male Breast
Embryology, the study of animal development, has been the subject of
scientific curiosity for thousands of years. Some of the earliest recorded
investigations were performed on the chick embryo by the Greek philosopher
Aristotle. The fusion of two gametes, the sperm and the egg, is termed
fertilization. The fertilized egg, or zygote, subsequently divides, differentiates,
and maturates into the neonate that is received at birth. This process
is extremely complex but proceeds in a regular, stepwise fashion to its
completion.
The mammary glands are a distinguishing feature of mammals and a primary
symbol of femininity in our culture. Breastfeeding provides nourishment
and passive immunity to offspring, promotes postpartum uterine involution,
and establishes an important bond between mother and infant. The mammary
gland begins development early in embryologic life and only culminates
in the postpartum lactation of the adult female. An understanding of
the development of the mammary gland is essential to anyone asked to
evaluate and treat aberrancies of such development.
Congenital breast malformations can be psychologically debilitating
to the individual. Accurate diagnosis, counseling, and treatment are
necessary to alleviate the sense of deformity and unattractiveness that
is often present. Proper timing of surgical intervention is necessary
to optimize functional, psychological, and aesthetic outcomes. This article
describes normal embryologic mammary gland development and the more common
congenital malformations that may confront the plastic surgeon.
The Integument
The integument consists of two layers, the epidermis and dermis, which
form a variety of structures that allow us to interact with our environment.
The epidermis is derived primarily from surface ectoderm but is colonized
by pigment-containing melanocytes of neural crest origin, antigen-processing
Langerhans cells of bone marrow origin, and pressure-sensing Merkel cells
of unclear origin. The dermis is derived primarily from mesoderm and
contains many blood vessels and sensory structures.
During the fourth week of embryologic development, the single cell thick
ectoderm and underlying mesoderm begin to proliferate and differentiate
into the definitive multi-layered skin present at birth. The specialized
structures formed by the skin, including teeth, hair, hair follicles,
fingernails, toenails, sebaceous glands, eccrine glands, apocrine glands,
and mammary glands, also begin to appear at approximately this period
in development. Teeth, hair, and hair follicles are formed by the epidermis
and dermis in concert, while fingernails and toenails are formed by the
epidermis alone.
Sebaceous glands, eccrine glands, apocrine glands, and mammary glands
are considered epidermal glands in that they develop as downgrowths or
diverticula of the epidermis into the dermis. Sebaceous glands, or holocrine
glands, are found over the entire surface of the body except the palms,
soles, and dorsum of the feet. They are largest and most concentrated
in the face and scalp where they are the site of origin of acne. The
normal function of sebaceous glands is to produce sebum, a group of complex
oils including triglycerides and fatty acid breakdown products, wax esters,
squalene, cholesterol esters, and cholesterol.
Eccrine sweat glands are found over the entire surface of the body except
the lips, external ear canal, and labia minora. They are most concentrated
in the palms and soles of the feet. The normal function of eccrine glands
is to produce the water and electrolyte component of sweat, which cools
the body by evaporation.
Apocrine sweat glands are similar in structure but not identical to
eccrine glands. They are concentrated in the axillae and anogenital regions.
They probably serve a vestigial sexual function, because they produce
odor and do not function prior to puberty. The mammary gland is considered
to be a modified and highly specialized type of apocrine gland.
The Embryologic Breast
Embryologic development of the mammary gland consists of a series of
highly ordered events involving interactions among a number of distinct
cell types. These interactions are regulated by an array of systemic
and local factors such as growth factors and hormones. Development is
initially identical among males and females of the same species.
During the fourth week of gestation, paired ectodermal thickenings termed
mammary ridges or milk lines develop on the ventral surface of the embryo
and extend in a curvilinear fashion convex towards the midline from the
axillae to the medial thigh. This is the first morphologic evidence of
mammary gland development. In normal human development, these ridges
disappear except at the level of the fourth intercostal space on the
anterior thorax, where the mammary gland subsequently develops. In other
species, such as cats and dogs, multiple paired mammary glands develop
along the mammary ridges in the chest, abdominal, and groin regions.
The number of paired glands varies greatly among mammalian species and
is related to the number of offspring in each litter.
During the fifth week of gestation, the remnant of the mammary ridge
ectoderm begins to proliferate and is termed the primary mammary bud.
This primary bud subsequently begins growth downward as a solid diverticulum
into the underlying dermis during the seventh week. By the 10th week,
the primary bud begins to branch, yielding secondary buds by the 12th
week, which eventually develop into the mammary lobules of the adult
breast.
This initial downgrowth and subsequent branching has been shown to occur
as the result of an inductive influence of the extracellular matrix of
the mesoderm on the primary mammary bud. This epithelial-mesenchymal
signaling is probably through paracrine and juxtacrine mechanisms where
the underlying mesoderm produces growth factors and hormones that interact
with receptors on the overlying ectodermal cells of the primary mammary
bud. The adipose tissue in the underlying mesoderm represents a significant
store of lipids for the production of hormones and growth factors, which
are then available to promote and regulate growth of the developing mammary
gland.
During the remainder of gestation, these buds continue lengthening and
branching. During the 20th week, small lumina develop within the buds
that coalesce and elongate to form the lactiferous ducts. The canalization
of the mammary buds with formation of the lactiferous ducts is induced
by placental hormones entering the fetal circulation. These hormones
include progesterone, growth hormone, insulinlike growth factor, estrogen,
prolactin, adrenal corticoids, and triiodothyronine. At term, approximately
15-20 lobes of glandular tissue have formed, each containing a lactiferous
duct. The supporting fibrous connective tissue, Cooper ligaments, and
fat of the mammary gland develop from the surrounding mesoderm.
The lactiferous ducts drain into retroareolar ampullae that converge
into a depressed pit in the overlying skin. Each of the 15-20 lobes of
the mammary gland has an ampulla with an orifice opening into this mammary
pit. Stimulated by the inward growth of the ectoderm, the mesoderm surrounding
this area proliferates, creating the nipple with circular and longitudinally
oriented smooth muscle fibers. The surrounding areola is formed by the
ectoderm during the fifth month of gestation. The areola also contains
other epidermal glands including glands of Montgomery, sebaceous glands
that serve to lubricate the areola.
The Neonatal Breast
At birth, the breast is composed of radially arranged mammary lobes
draining via lactiferous ducts into ampullae that empty onto the nipple.
These rudimentary mammary glands are identical in males and females.
The nipple appears as a small pit in the center of a thickened areola
containing a few glands of Montgomery. Shortly after birth, the nipples
become everted from proliferation of the surrounding mesoderm, and the
areolae develop a slight increase in pigmentation. The development of
erectile tissue in the nipple areolar complex causes the nipple to protrude
even further upon stimulation.
Failure of the nipples to evert may indicate the presence of inverted
nipples, which is usually caused by fibrous bands and a hypoplastic ductal
system tethering the nipple in the inverted position. This condition
is familial in 50% of patients, may occur in males and females, and is
only clinically significant in that it may present a muchanical problem
for women attempting to breastfeed an infant. The new onset of inverted
nipples in adolescence or adulthood may indicate infection or malignancy.
Falling levels of maternal estrogens in the neonatal bloodstream stimulate
the neonatal pituitary hypophysis to produce prolactin. This results
in unilateral or bilateral breast enlargement in as many as 70% of neonates.
Histologically, this appears as hypertrophy of the ductal system. Acini
appear and an increase in vascularity of the gland is observed. This
is often accompanied by the secretion of witch's milk, a cloudy fluid
similar to colostrum consisting mainly of water, fat, and cellular debris.
These changes occur equally among male and female neonates and regress
spontaneously within several weeks as neonatal prolactin production declines
and the glands become quiescent. However, attempting to strip the breasts
of their witch's milk, as advocated by some superstitions, can lead to
a persistent hyperplastic secretory state lasting many months.
The Male Breast
Embryologic breast development and the transient secretory phase of
the neonatal breast occur in male and female breasts. However, important
developmental differences should be noted. Although the early stages
of development are independent of sex steroid hormones, the mammary glands
become extremely responsive to their hormonal environment. The presence
of testosterone and its binding to mesodermal receptors leads to the
normal involution of the male mammary gland. However, in testicular feminization
syndrome, where circulating testosterone but an absence of testosterone
receptors occurs, the individual develops a female phenotype, including
typical female breast development.
Approximately two thirds of males develop some degree of hyperplasia
of the breasts, or gynecomastia, during puberty. This condition persists
for several months to several years and will spontaneously resolve in
all but approximately 7%. The etiology is usually a decreased ratio of
testosterone to estradiol, and when testicular function becomes dominant,
the hypertrophy resolves. Although temporary in most instances, this
condition can be psychologically devastating to a teenage boy who may
be teased by his peers and, as a result, becomes unwilling to change
in gym class or remove his shirt at the pool or beach.
During this period, the patient or his family may seek consultation
and treatment. Reassurance that the condition is most likely temporary
provides psychological support and hopefully avoids premature surgical
intervention. Use of certain drugs, namely H2 blockers, anabolic steroids,
marijuana, and heroin, can prolong the presence of gynecomastia in this
population. Gynecomastia also is seen in approximately 40% of males with
Klinefelter syndrome (trisomy of the sex chromosomes [XXY]).
Treatment of gynecomastia without underlying cause that does not resolve
spontaneously within several years is relatively simple. Because of the
elasticity of the skin in this population, liposuction alone or a combination
of liposuction with direct subareolar excision techniques is generally
successful. Older patients with less elastic skin may require more standard
breast reduction techniques. Obese patients may benefit from preoperative
weight loss, but still require excision of some sort. Gynecomastia and
its treatment is discussed elsewhere on this website.
Supernumerary breast tissue and the absence of breast tissue also occur
in males and are discussed with other congenital breast malformations
in this article.
The Female Breast
Development of the mammary glands is initiated during embryologic life
but is only complete in the postpartum lactation of the adult female.
After the transient secretion stimulated by prolactin production in the
neonate, the mammary glands, with their relatively simple architecture,
remain quiescent until puberty. During this period, the supporting stromal
structures and ducts enlarge in proportion to the increase in body size
of the individual, but no lobular development occurs.
Thelarche, the rapid growth that occurs at the onset of puberty, is
primarily from deposition of fat and development of periductal connective
tissue, but elongation and thickening of the ductal system also occurs
at this stage. Ductal growth occurs under the influence of circulating
estrogens, growth hormone, and prolactin but is independent of progesterone.
The development initiated at the onset of puberty is generally complete
by age 20 years.
If pregnancy occurs, the glands complete their differentiation and reach
functional maturity with the intralobular-branched ducts forming buds
that become secretory alveoli. This occurs under the influence of sustained
increases in the levels of circulating progesterone, estrogens, prolactin,
and placental lactogen. The epithelial cells of the alveoli begin to
accumulate the cytoplasmic organelles necessary to sustain lactation
in the postpartum period.
Following parturition, lactation proceeds as a response to environmental
stimuli and the withdrawal of circulating progesterone. Prolactin, originating
in the anterior pituitary, and somatomammotropin, originating in the
placenta, stimulate the alveolar epithelium to produce and secrete milk
proteins, primarily casein and alpha-lactalbumin, and lipids. In response
to suckling or crying, oxytocin produced by the hypothalamus but released
by the posterior pituitary causes contraction of myoepithelial cells
surrounding the alveoli, which expels the milk to assist the suckling
child.
With cessation of nursing, milk production also ceases because of a
decrease in circulating prolactin and the inhibitory effects of nonexpelled
milk. The alveoli then return to their prior nonfunctioning state. By
age 40 years, the mammary glands begin to atrophy. During and after menopause,
the altered hormonal environment leads to a senescent state, with involution
of the glandular component and replacement with connective tissue and
fat.
Congenital Breast Malformations
Congenital breast malformations range in severity from the relatively
minor to major chest wall deformities. Minor malformations may not even
be recognized, while major deformities may cause significant functional,
psychological, and aesthetic concerns. The affected individual may present
for consultation at any age, often early in childhood as a result of
parental concern. It is important to be able to counsel the patient and
his or her family regarding the nature of the problem, its prognosis
for future development, and the appropriate indications and timing of
surgical intervention. These malformations generally fall into one of
two categories, the presence of supernumerary breast tissue or the absence
or underdevelopment of breast tissue.
The presence of supernumerary breast tissue indicates incomplete involution
of the milk line, resulting in the formation of accessory mammary tissue
from the redundant clusters of ectopic primordial breast cells. This
occurs in 2-6% of females and 1-3% of males. Approximately one third
of affected individuals have more than one site of supernumerary breast
tissue development. Most of this accessory breast tissue has no physiologic
significance, but some may enlarge with the onset of puberty, pregnancy,
or lactation, and can be the site of breast carcinoma.
Approximately 67% of accessory breast tissue occurs in the thoracic
or abdominal portions of the milk line, often just below the inframammary
crease and more often on the left side of the body. Another 20% occurs
in the axilla. The remaining locations include anywhere along the milk
line or in the buttock, back, face, and neck. Supernumerary tissue present
in any location other than along the milk line represents a migratory
arrest of breast primordium during chest wall development.
In 1915, Kajava published a classification system for supernumerary
breast tissue that remains in use today. Class I consists of a complete
breast with nipple, areola, and glandular tissue. Class II consists of
nipple and glandular tissue but no areola. Class III consists of areola
and glandular tissue but no nipple. Class IV consists of glandular tissue
only. Class V consists of nipple and areola but no glandular tissue (pseudomamma).
Class VI consists of a nipple only (polythelia). Class VII consists of
an areola only (polythelia areolaris). Class VIII consists of a patch
of hair only (polythelia pilosa).
The most common form of supernumerary breast tissue is polythelia, the
presence of more than 2 nipples on an individual. More than 90% of supernumerary
nipples occur in the inframammary region. This condition is present in
2-5% of the general population, although many additional patients may
go undiagnosed because the nipple is often confused for a nevus or other
benign skin lesion because of its diminutive size. Males and females
have an overall equal incidence, but differences are observed within
ethnic groups. For example, polythelia is present in 5% of Japanese females
but only 1.6% of Japanese males. Differences also exist among ethnic
groups. Polythelia occurs less frequently in Caucasians (0.6%) than in
blacks (3.5%). Most cases are sporadic, but approximately 6% are familial
and are believed to represent an autosomal dominant trait with variable
penetrance.
The image below was a supernumerary nipple found just beneath the inframammary
crease of the right breast of a young woman. This was removed for cosmetic
reasons.
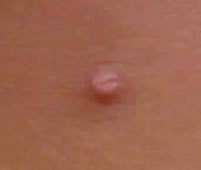
A correlation exists between renal disease and polythelia. Nephrologic
abnormalities such as cysts, duplications, or unilateral renal agenesis
have been found in 14.5% of sporadic cases and 32.1% of familial cases
compared to 1-2% of the general population. Approximately 19% of patients
with renal adenocarcinoma have polythelia, and 16.5% of patients with
end-stage renal disease have polythelia. Considering the significant
incidence of congenital and acquired renal disorders in patients with
polythelia, patients should be aware of the need for regular physical
examination and urinalysis. Any abnormality noted should alert the physician
to the need for a renal ultrasound.
Polymastia, the presence of accessory glandular tissue, is the second
most common form of supernumerary breast tissue, occurring in 1-2% of
the female population. Various forms exist, as described by Kajava Classes
I through IV, but most commonly the nipple and areola are absent or rudimentary.
The most common location is in the axilla, where they may present as
axillary fullness responsive to hormonal cycles of menstruation, pregnancy,
or lactation. The second most common location is in the inframammary
region, similar to polythelia. Most cases are sporadic, but this condition
also has been observed as a heritable trait.
The presence of supernumerary tissue can be psychologically disturbing
to adolescents. Excision is recommended prior to puberty or at any age
when the condition is recognized and becomes of concern to the individual.
The absence or underdevelopment of breast tissue is less common than
the presence of supernumerary tissue. These conditions may be unilateral
or bilateral and result from partial or complete underdevelopment of
the mammary bud. The most severe form is amastia, the complete absence
of glandular tissue, nipple, and areola. Hypoplasia, the presence of
very small rudimentary breasts, is the most common form. Amastia and
hypoplasia may be associated with scalp defects, ear abnormalities, renal
hypoplasia, and cataracts in patients with the rare autosomal dominant
Finlay-Marks syndrome. Hypoplasia also may occur in patients with Turner
syndrome (ovarian agenesis), congenital adrenal hyperplasia, and delayed
menarche where the administration of oral estrogen therapy usually promotes
glandular development.
Aplasia, the absence of glandular tissue in the presence of a nipple
and areola, is most commonly encountered in Poland syndrome, first described
in 1841. This condition is often accompanied by musculoskeletal deformities
of the chest wall and ipsilateral upper extremity and is discussed in
a separate article. Athelia, absence of the nipple and areola in the
presence of glandular tissue, is the most rare of these conditions. It
is infrequently seen as an isolated defect except in ectodermal dysplasia
syndromes.
The goal of surgical therapy in these patients is to achieve breast
symmetry. This may best be performed in late adolescence when the contralateral
breast has reached its mature size and shape. However, earlier intervention
may be indicated in the patient with a significant sense of deformity
that is adversely affecting body image. Techniques to achieve symmetry
may include prosthetic devices such as implants or expanders, autologous
tissue such as the latissimus dorsi or rectus abdominis muscles, or both.
Conclusion
The mammary gland is a complex organ that begins development early in
gestation. The extensive research currently being performed on the human
genome, molecular biology, and growth factors will certainly contribute
to the further elucidation of the embryologic factors involved in the
formation, differentiation, and development of the mammary gland. This
may provide important insights into the cause, treatment, and potential
prevention of mammary gland abnormalities, such as congenital malformations
and breast cancer. Given that breast cancer strikes 10% of women in the
US and congenital malformations occur in 2-6%, these developments may
have enormous implications for the future of medicine.
Bibliography
- Carlson BM: Integumentary, skeletal, and muscular systems. Human
Embryology and Developmental Biology 1994; 1st edition: 153-181.
- Casey HD, Chasan PE, Chick LR: Familial polythelia without associated
abnormalities. Ann Plast Surg 1996; 36: 101-104.
- Denk MJ: Topics in pediatric plastic surgery. Pediatr Clin North
Am 1998; 45(6): 1479-1506.
- Forsyth IA: The mammary gland. Baillieres Clin Endocrinol Metab 1991;
5(4): 809-832.
- Georgiade NG, Georgiade GS, Riefkohl R: Esthetic breast surgery.
Plastic Surgery 1990; 5: 3839-3896.
- Gilbert SF: Cell interactions at a distance: hormones as mediators
of development. Developmental Biology 1997; 5th edition: 733-772.
- Hovey RC, McFadden TB, Akers RM: Regulation of mammary gland growth
and morphogenesis by the mammary fat pad:a species comparison. J Mammary
Gland Biol Neoplasia 1999; 4(1): 53-68.
- Kajava Y: The proportions of supernumerary nipples in the Finnish
population. Duodecim 1915; 31: 143-170.
- Kamalati T, Niranjan B, Yant J et al: HGF/SF in mammary epithelial
growth and morphogenesis:in vitro and in vivo models. J Mammary Gland
Biol Neoplasia 1999; 4(1): 69-77.
- McCarty KS Jr, Carpenter SA, Georgiade GS: The breast: embryology,
anatomy, and physiology. Aesthetic Surgery of the Breast 1990; 1st
edition: 3-18.
- Medina D: The mammary gland: a unique organ for the study of development
and tumorigenesis. J Mammary Gland Biol Neoplasia 1996; 1(1): 5-19.
- Moore, KL, Persuad, TVN: The integumentary system. Before We Are
Born: Essentials of Embryology and Birth Defects 1998; 5th edition:
481-496.
- Plessis G, Le Treust M, Le Merrer M: Scalp defect, absence of nipples,
ear anomalies, renal hypoplasia:another case of Finlay-Marks syndrome.
Clin Genet 1997; 52: 231-234.
- Robinson GW, Karpf AB, Kratochwil K: Regulation of mammary gland
development by tissue interaction. J Mammary Gland Biol Neoplasia 1999;
4(1): 9-19.
- Romrell LJ, Bland KI: Anatomy of the breast, axilla, chest wall,
and related metastatic sites. The Breast: Comprehensive Management
of Benign and Malignant Diseases 1991; 1st edition: 17-35.
- Rush BF Jr: Breast. Principles of Surgery 1989; 5th edition: 549-580.
- Salomon, DS, Bianco C, De Santis, M: Cripto:a novel epidermal growth
factor (EGF)-related peptide in mammary gland development and neoplasia.
BioEssays 1999; 21: 61-70.
- Urbani CE, Betti R: Sporadic unilateral intra-areolar polythelia.
Acta Derm Venereol 1995; 76: 156.
- Walden PD, Ruan W, Feldman M et al: Evidence that the mammary fat
pad mediates the action of growth hormone in mammary gland development.
Endocrinology 1998; 139(2): 659-1292.
For more information, please call our office for a complimentary consultation
((954) 630-2009), or you may email Dr. Revis at the address below.
Email: DrDonRevis@hotmail.com
|





